Moderna Covid-19 vaccine gets Phase 2 study green light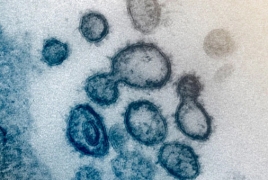 May 8, 2020 - 12:36 AMT PanARMENIAN.Net - The promising coronavirus vaccine developed by Cambridge-based company Moderna was cleared by the FDA for a phase 2 study, a step that will push the much needed vaccine forward, Boston Herald reports. Moderna Therapeutics, a Cambridge, Mass.-based biotech company co-founded by Armenian entrepreneur Noubar Afeyan, shipped the first batches of its Covid-19 vaccine in February. “The imminent Phase 2 study start is a crucial step forward as we continue to advance the clinical development of mRNA-1273, our vaccine candidate against SARS-CoV-2,” said Stephane Bancel, Moderna’s chief executive officer in a press release issued by the company on Thursday. Moderna has a goal of starting a pivotal phase 3 study in the summer and could have its first biologics license application approved as soon as next year, Bancel’s statement said. “We are accelerating manufacturing scale-up and our partnership with Lonza puts us in a position to make and distribute as many vaccine doses of mRNA-1273 as possible, should it prove to be safe and effective,” said Bancel. The foreign ministers of Armenia and Azerbaijan, Ararat Mirzoyan and Jeyhun Bayramov, have arrived in Washington. The CSTO budget for the current year requires adjustments due to the refusal of Yerevan to pay their share of contributions. Six total incidents have burned 19 old-growth trees. Friday night 8 trees were torched along the beautiful main entrance. The EU does not intend to conduct military exercises with Armenia, Lead Spokesperson for EU Foreign Affairs and Security Policy Peter Stano says. Partner news |