Deployment of second Ebola vaccine would not be quick fix: experts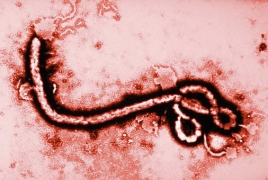 July 26, 2019 - 14:08 AMT PanARMENIAN.Net - The resignation of Congo’s health minister in the midst of the country’s worst Ebola outbreak could clear the way for a second experimental vaccine to be deployed. But the new shot would likely take months to win the trust of frightened locals and show results, health officials say, according to Reuters. Oly Ilunga, who opposed using the vaccine developed by U.S. pharmaceutical giant Johnson & Johnson, resigned as minister on Monday after being bumped off the Ebola response team. The World Health Organization recommended the two-dose shot to complement a vaccine by U.S. drugmaker Merck, which has proved highly protective but is in relatively short supply. Proponents, including medical charity Medecins Sans Frontieres (Doctors Without Borders) and the Wellcome Trust, said the new vaccine could be deployed to areas not yet affected by Ebola to create a firewall against the virus, which the WHO declared an international health emergency last week. But Ilunga said the J&J vaccine had not been proven effective and could confuse people in eastern Democratic Republic of Congo, where wild rumours are hampering the response. “Congolese have the right to have the gold standard, the best vaccine,” he told Reuters on Thursday, in his first public comments since resigning. “They don’t need to be the subject of experimentation.” “You can’t have a group of promoters, producers of the vaccine (and) university researchers wanting to introduce the vaccine without contacting the health authorities,” he said, without elaborating further. Authorities said a total of 192 Azerbaijani troops were killed and 511 were wounded during Azerbaijan’s offensive. In 2023, the Azerbaijani government will increase the country’s defense budget by more than 1.1 billion manats ($650 million). The bill, published on Monday, is designed to "eliminate the shortcomings of an unreasonably broad interpretation of the key concept of "compatriot". The earthquake caused a temporary blackout, damaged many buildings and closed a number of rural roads. Partner news |