RIA: EU accepts Russia’s application for Sputnik V approval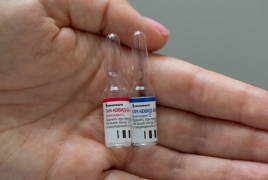 February 9, 2021 - 16:51 AMT PanARMENIAN.Net - The European Union has approved Russia’s application to register its Sputnik V coronavirus vaccine in the bloc, the state-run RIA Novosti news agency reported Tuesday, February 9. The Russian Direct Investment Fund (RDIF), which is funding and marketing Sputnik V, filed for EU registration of the jab on Jan. 29. The European Medicines Agency (EMA) had concluded its scientific opinion procedure for the vaccine on Jan. 19. It is now up to the EMA to grant conditional marketing authorization to the vaccine so it can be centrally supplied to the EU, The Moscow Times says. The EU has already authorized coronavirus vaccines developed by Pfizer/BioNTech, Moderna and AstraZeneca. In addition, it has signed vaccine supply contracts with Johnson & Johnson, Sanofi-GSK and CureVac. However, the bloc has grappled with a slower-than-expected vaccination rollout due to supply shortfalls. Minister of Health Anahit Avanesyan said on February 3 Armenia was negotiating the purchase of the Russian Sputnik V vaccine against the coronavirus. Authorities said a total of 192 Azerbaijani troops were killed and 511 were wounded during Azerbaijan’s offensive. In 2023, the Azerbaijani government will increase the country’s defense budget by more than 1.1 billion manats ($650 million). The bill, published on Monday, is designed to "eliminate the shortcomings of an unreasonably broad interpretation of the key concept of "compatriot". The earthquake caused a temporary blackout, damaged many buildings and closed a number of rural roads. Partner news |